Coronavirus (COVID-19)
National and International Guidance Documents
Public Health Agency of Canada CORONAVIRUS HOTLINE 1-833-784-4397
Canadian and International guidelines, policies and standards that Infection Prevention and Control professionals may use to support their own documentation and best practices during the COVID-19 Pandemic.
Quick Index
- Government of Canada
- Guidance in Healthcare Settings
- COVID-19 Guidance During Pregnancy
- Laboratory and Supply Chain Guidance
- Guidance on Personal Protective Equipment and Alcohol-Based Hand Sanitizer
- Vaccine Guidance
- National Advisory Committee on Immunization (NACI)
- Public Health Guidance
- School and Community Guidance
- Awareness Resources
- CDC
- WHO
- APIC
- Other
Government of Canada
- PHAC COVID-19 Vaccine Newsletter registration: HC-PHAC.PublicEducation-Educationpublique.SC-ASPC@hc-sc.gc.ca.
- SARS-CoV-2 variants: National definitions, classifications and public health actions
- Mathematical modelling and COVID-19
- Federal/Provincial/Territorial Public Health Response Plan for Ongoing Management of COVID-19
- COVID-19: Current Situation
- COVID-10 Symptoms and Treatments
- COVID-19 signs, symptoms and severity of disease: A clinician guide
- Respiratory infectious diseases: How to reduce the spread with personal protective measures
- COVID-19 Prevention and risks
- Coronavirus disease (COVID-19): Canada’s Response
- COVID-19: Travel, testing and borders
- From risk to resilience: An equity approach to COVID-19 -Chief Public Health Officer of Canada's Report on the State of Public Health in Canada 2020
- Race-Based Data Collection and Health Reporting
- Pan-Canadian COVID-19 Testing and Screening Guidance: Technical guidance and implementation plan
- Pan-Canadian virtual care priorities in response to COVID-19
- Mental Illness during the Pandemic: Survey on COVID-19 and Mental Health
- COVID-19 and deaths in older Canadians: Excess mortality and the impacts of age and comorbidity
- Frequency and impact of longer-term symptoms following COVID-19 in Canadian adult
- Impact of COVID-19 in adults with chronic conditions: Emergency department visits
- Impacts of COVID-19 on Canadian nursing homes and seniors’ homes in 2021
- COVID-19 mortality among racialized populations in Canada and its association with income
- Unmet health care needs during the pandemic and resulting impacts among First Nations people living off reserve, Métis and Inuit
- Infographic: Examining how changes in alcohol and cannabis consumption varied by experiences of stigma during the COVID-19 pandemic in Canada
- Inequalities in the mental health of adults before and during the COVID-19 pandemic: data tool
- Social inequalities in COVID-19 deaths in Canada
COVID-19 Guidance in Healthcare Settings
- COVID-19 Pandemic Guidance for the Health Care Sector
- COVID-19 for Health Professionals
- Updated guidance for infection prevention and control in health care settings when COVID-19 is suspected or confirmed – April 2024 (French)
COVID-19 Variants of Concern -Guidelines, Policies and Standards
- COVID-19 epidemiology update: Testing and variants (PHAC)
- COVID-19 Variants of Concern (VOCs) (PHO)
- Variants COVID-19 (AHS)
- Tracking SARS-CoV-2 Variants (WHO)
- COVID-19 Variants (BCCDC)
- SARS-CoV-2 variants: National definitions, classifications and public health actions (PHAC)
COVID-19 Guidance During Pregnancy
- Statement on Pregnant Workers during the COVID-19 Pandemic (SOGC)
- COVID-19 and Pregnancy (SOGC)
- Statement on COVID-19 Vaccination in Pregnancy (SOGC)
Laboratory and Supply Chain Guidance during the COVID-19 Pandemic
COVID-19 Guidance on Personal Protective Equipment (PPE) and Alcohol-Based Hand Sanitizer (ABHR)
- COVID-19 mask use: Advice for community settings
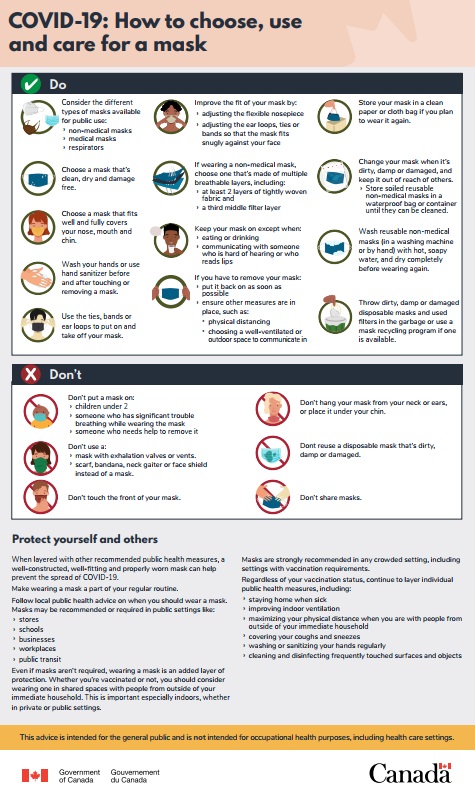
- COVID-19 mask use: How to choose, use and care for a mask
Vaccine Guidance
- COVID-19: How vaccines are developed (Video)
- Vaccine development and approval in Canada
- Guidance for market authorization requirements for COVID-19 drugs: Overview
- Health Canada authorizes Medicago COVID-19 vaccine for adults 18 to 64 years of age
- Pfizer-BioNTech Comirnaty COVID-19 vaccine
- Health Canada authorizes second bivalent COVID-19 booster targeting the Omicron BA.4/5 variants
National Advisory Committee on Immunization (NACI)
- NACI Statement: Interim guidance on booster COVID-19 vaccine doses in Canada
- NACI: Summary of the updated vaccine statement -MRNA COVID-19 Vaccines and Myocarditis
- NACI Statement: Interim guidance on booster COVID-19 vaccine doses in Canada
- NACI: Extended dose intervals for COVID-19 vaccines to optimize early vaccine rollout and population protection in Canada in the context of limited vaccine supply
- NACI: Summary of Extended Dose Intervals Statement of April 7, 2021
- NACI Summary -Recommendation on the use of the PfizerBioNTech COVID-19 Vaccine in children 5-11 years of age
- NACI Rcommendation on the use of the Pfizer-BioNTech COVID-19 vaccine (10 mcg) in children 5-11 years of age
- NACI Statement: Rapid response: Updated guidance on COVID-19 vaccination timing for individuals previously infected with SARS-CoV-2
- NACI Summary: Updated guidance on COVID-19 vaccination timing for individuals previously infected with SARS-CoV-2
- NACI Recommendations on the use of Novavax Nuvaxovid COVID-19 vaccine (PDF)
- Summary of the NACI statement of February 17, 2022: Recommendations on the use of Novavax Nuvaxovid COVID-19 vaccine
- NACI Statement: Recommendations on the use of Medicago COVID-19 vaccine (Covifenz
- Summary of the NACI statement of March 11, 2022
- NACI Statement: Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 to 11 years of age
- Summary of the NACI statement of March 17, 2022: Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 to 11 years of age
- NACI Statement: Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 months to 5 years of age
- Summary of NACI statement of July 14, 2022: Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 months to 5 years of age
- NACI Recommendations on the use of a first booster dose of Pfizer-BioNTech Comirnaty COVID-19 vaccine in children 5 to 11 years of age
- NACI Summary: Recommendations on the use of a first booster dose of Pfizer-BioNTech Comirnaty COVID-19 vaccine in children 5 to 11 years of age
- NACI Statement: Updated guidance on COVID-19 vaccines for individuals who are pregnant or breastfeeding
- NACI Summary: Updated guidance on COVID-19 vaccines for individuals who are pregnant or breastfeeding
- NACI Updated recommendations on the use of COVID-19 vaccine booster doses in children 5 to 11 years of age and concurrent vaccine administration
- Summary of NACI statement of December 9, 2022: Updated recommendations on the use of COVID-19 vaccine booster doses in children 5 to 11 years of age and concurrent vaccine administration
- NACI Guidance on an additional COVID-19 booster dose in the spring of 2023 for individuals at high risk of severe illness due to COVID-19
- Summary of NACI statement of March 3, 2023: NACI Guidance on an additional COVID-19 booster dose in the spring of 2023 for individuals at high risk of severe illness due to COVID-19
- Summary of NACI statement of May 3, 2024: Guidance on the use of COVID-19 vaccines during the fall of 2024
COVID-19 Awareness Resources
-
Coronavirus disease (COVID-19): Awareness resources (Posters and Videos)
- People who are at risk of more severe disease or outcomes from COVID-19
- COVID-19 antibody (serology) testing: Information for patients
- COVID-19: We're doing this for this (closed captions in other languages)
- COVID-19: How vaccines are developed
- Vaccine development and approval in Canada
Centers for Disease Control and Prevention
- CDC - Coronavirus COVID-19
- How COVID-19 Spreads
- Testing for COVID-19
- COVID-19 Treatments and Medications
- Stay Up to Date with COVID-19 Vaccines Including Boosters
- Strategies for Optimizing the Supply of PPE
World Health Organization/Organisation mondiale de la santé
- WHO -Coronavirus COVID-19 Outbreak
- COVID-19 Global Cases (WHO)
- Global technical consultation report on proposed terminology for pathogens that transmit through the air
- Infection prevention and control in the context of COVID-19: a guideline
- Transmission of SARS-CoV-2: implications for infection prevention precautions
- WHO Online Course: Infection Prevention and Control (IPC) for Novel Coronavirus (COVID-19)
- WHO Rational use of personal protective equipment for coronavirus disease (COVID-19)
- Coronavirus disease (COVID-19) technical guidance: Infection prevention and control
- Criteria for releasing COVID-19 patients from isolation
- WHO Rational use of personal protective equipment for coronavirus disease (COVID-19)
- Novel coronavirus (COVID-19) Video
- Situation Reports
Association for Professionals in Infection Control and Epidemiology
European Centre for Disease Control
- Algorithm for management of contacts of probable or confirmed COVID-19 cases
- Advice to healthcare workers: management of patients with COVID-19 infection
- Advice for travellers: outbreak of a novel coronavirus COVID-19
- Risk assessment: outbreak of acute respiratory syndrome associated with a novel coronavirus
Other Guidelines
- COVID-19: Recommendations for School Reopening (Sick Kids)
- COVID-19 and Pregnancy (SOGC)
- Briefing Note on Addressing Mental Health and Psychosocial Aspects of COVID-19 Outbreak (United Nations Office for the Coordination of Humanitarian Affairs)
- ASHRAE Position Document on Infectious Aerosols (ASHRAE)
- Policy Guidance for the Reintegration of Caregivers as Essential Care Partners (CFHI & CPSI)
- Statement on Pregnant Workers during the COVID-19 Pandemic (SOGC)
Dentistry
- Evidence to support safe return to clinical practice by oral health professionals in Canada during the COVID-19 pandemic: A report prepared for the Office of the Chief Dental Officer of Canada (PHAC)
- COVID-19 (Canadian Dental Association)
- COVID-19 Recommendations (Canadian Dental Assistants Association)
- Recommendations for Dental Hygienists and Canadian Dental Assistants During COVID-19 Outbreak (Canadian Dental Assistants Association)
Date Modified: April 22, 2024





