Quick Index
COVID-19 Vaccination and Treatment Guidance
Policies and standards related to the COVID-19 vaccines that Infection Prevention and Control professionals may use to support their own documentation and best practices during the COVID-19 Pandemic.
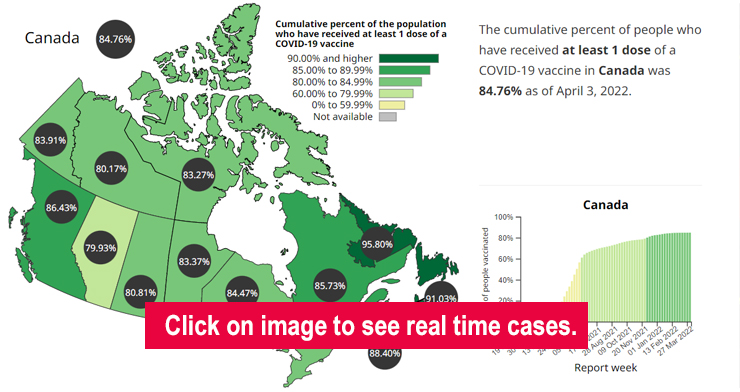
COVID-19 Vaccination Tracker
For more information on vaccination rates in Canada and globally refer to:
COVID-19 Vaccine Guidance
- Planning guidance for immunization clinics for COVID-19 vaccines: Introduction
- Statement from the Council of Chief Medical Officers of Health (CCMOH): Update on the Use of COVID-19 Vaccine Boosters and on COVID-19 Vaccines and the Risk of Myocarditis and Pericarditis
- Statement from the Council of Chief Medical Officers of Health (CCMOH) on the importance of staying up to date with COVID-19 vaccines
- COVID-19 vaccination highly effective in individuals with immune-related inflammatory diseases
- COVID-19 Immunity Task Force: SeroTracker
National Advisory Committee on Immunization (NACI) COVID-19 Guidance
- NACI Statement: Interim guidance on booster COVID-19 vaccine doses in Canada
- NACI: Rapid Response Updated recommendation on the use of authorized COVID-19 vaccines in individuals aged 12 years and older in the context of myocarditis and pericarditis reported following mRNA COVID-19 vaccine
- NACI: Guidance on booster COVID-19 vaccine doses in Canada
- NACI: Summary of the updated vaccine statement -MRNA COVID-19 Vaccines and Myocarditis
- NACI Statement: Interim guidance on booster COVID-19 vaccine doses in Canada
- NACI: Extended dose intervals for COVID-19 vaccines to optimize early vaccine rollout and population protection in Canada in the context of limited vaccine supply
- NACI: Summary of Extended Dose Intervals Statement of April 7, 2021
- Summary of the NACI Statement -Recommendation on the use of the PfizerBioNTech COVID-19 Vaccine in children 5-11 years of age
- NACI Recommendation on the use of the Pfizer-BioNTech COVID-19 vaccine (10 mcg) in children 5-11 years of age
- NACI: Rapid Response: Guidance on the use of booster COVID-19 vaccine doses in adolescents 12 to 17 years of age
- NACI: Summary of the NACI rapid response of January 28, 2022
- NACI Statement: Recommendations on the use of Medicago COVID-19 vaccine (Covifenz
- Summary of the NACI statement of March 11, 2022
- NACI Statement: Initial guidance on a second booster dose of COVID-19 vaccines in Canada
- NACI Statement: Updated guidance on a first booster dose of COVID-19 vaccines in Canada
- NACI Summary: Updated guidance on a first booster dose of COVID-19 vaccines in Canada
- NACI Statement: Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 months to 5 years of age
- Summary of NACI statement of July 14, 2022: Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 months to 5 years of age
- Interim guidance on planning considerations for a fall 2022 COVID-19 vaccine booster program in Canada
- Summary of NACI of June 29, 2022 -Interim guidance on planning considerations for a fall 2022 COVID-19 vaccine booster program in Canada
- NACI Statement: Updated guidance on COVID-19 vaccines for individuals who are pregnant or breastfeeding
- NACI Summary: Updated guidance on COVID-19 vaccines for individuals who are pregnant or breastfeeding
- NACI Statement: Recommendations on the use of Pfizer-BioNTech Comirnaty (3 mcg) COVID-19 vaccine in children 6 months to 4 years of age
- NACI Summary: Recommendations on the use of Pfizer-BioNTech Comirnaty (3 mcg) COVID-19 vaccine in children 6 months to 4 years of age
- Summary of National Advisory Committee on Immunization (NACI) updates of November 3, 2022: Recommendations on the use of Moderna Spikevax BA.4/5 bivalent mRNA (50 mcg) COVID-19 booster vaccine in adults
- Summary of NACI updates: Recommendations on the use of Moderna Spikevax BA.4/5 bivalent mRNA (50 mcg) COVID-19 booster vaccine in adults
- Summary of NACI statement : Updated recommendations on the use of COVID-19 vaccine booster doses in children 5 to 11 years of age and concurrent vaccine administration
- NACI Updated recommendations on the use of COVID-19 vaccine booster doses in children 5 to 11 years of age and concurrent vaccine administration
- NACI Guidance on an additional COVID-19 booster dose in the spring of 2023 for individuals at high risk of severe illness due to COVID-19
- Summary of NACI statement: NACI Guidance on an additional COVID-19 booster dose in the spring of 2023 for individuals at high risk of severe illness due to COVID-19
- NACI Guidance on the use of COVID-19 vaccines in the fall of 2023
- Summary of NACI statement: Guidance on the use of COVID-19 vaccines in the fall of 2023
- NACI Guidance on the use of COVID-19 vaccines for 2025 to summer 2026
- Summary of NACI statement of January 10, 2025: Guidance on the use of COVID-19 vaccines for 2025 to summer 2026
Vaccine Development and Rollout
- Canada’s COVID-19 vaccine supply and donation strategy
- COVID-19: How vaccines are developed
- Vaccine development and approval in Canada
- Vaccines and treatments for COVID-19: Vaccine rollout
Vaccine Storage Guidance and Administration Guidance
- Vaccines and treatments for COVID-19: Vaccine rollout
- Vaccine safety, concerns and possible side effects